Clinical Resources
Materials for Providers
Alphabetical by Title
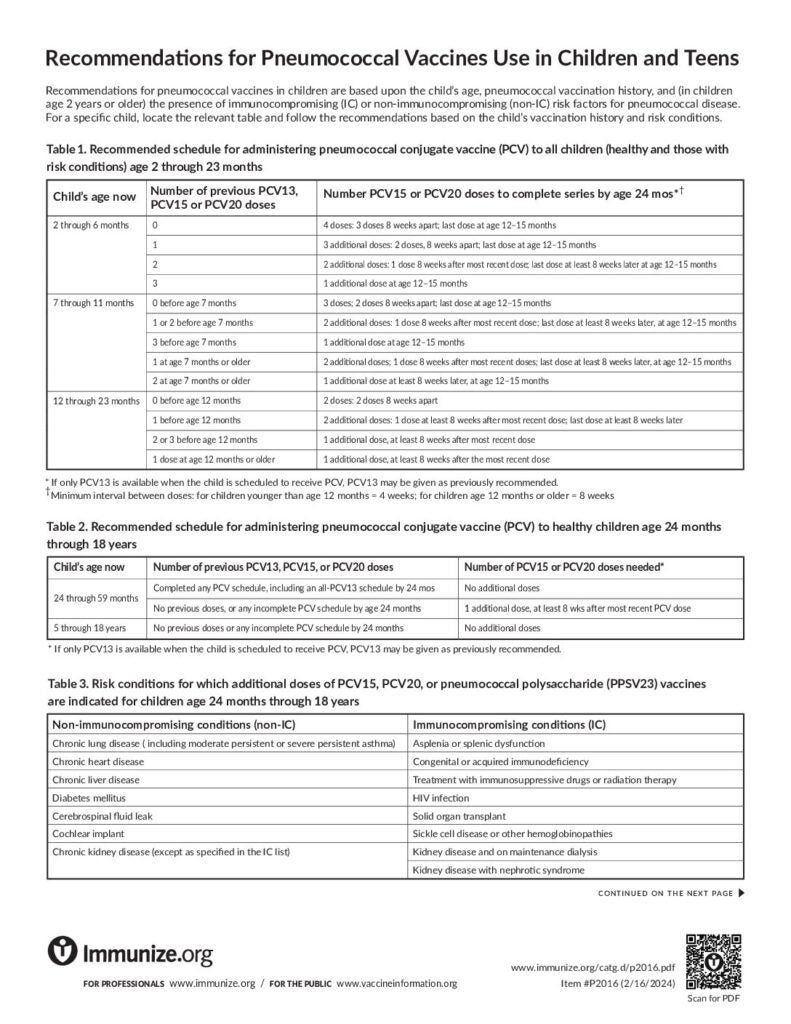
Recommendations for Pneumococcal Vaccines Use in Children and Teens
This piece gives recommendations for pneumococcal vaccines use in children and teens who are healthy or have risk factors.
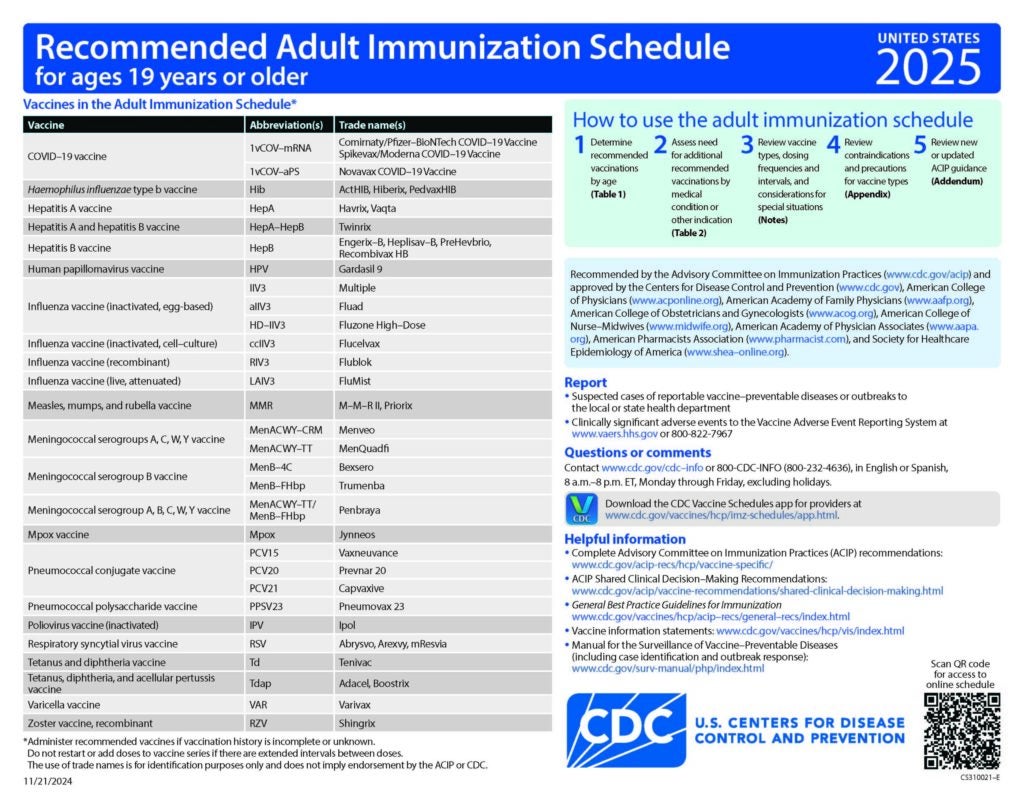
Standing Orders for Administering Pneumococcal Vaccines to Adults
Eligible healthcare professionals may vaccinate adults who meet any of the criteria on this form
Ask the Experts
CDC · FDA · State
ACIP Recommendations
Current Recommendations
Use of 15-Valent Pneumococcal Conjugate Vaccine Among U.S. Children: Updated Recommendations of the Advisory Committee on Immunization Practices—United States, 2022
MMWR, September 16, 2022, 71(37);1174–1181
Use of 15-Valent Pneumococcal Conjugate Vaccine and 20-Valent Pneumococcal Conjugate Vaccine Among U.S. Adults: Updated Recommendations of the Advisory Committee on Immunization Practices
MMWR, January 28, 2022, 71 (4); 109-117
Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged 65 Years and older: Updated Recommendations of the Advisory Committee on Immunization Practices
MMWR, November 22, 2019; 68(46):1069–1075
Intervals Between PCV13 and PPSV23 Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)
MMWR, September 4, 2015; 64(34):944-947
Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged >65 Years: Recommendations of the Advisory Committee on Immunization Practices (ACIP)
MMWR, September 19, 2014; 63(37):822-825
Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine Among Children Aged 6–18 Years with Immunocompromising Conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP)
MMWR, June 28, 2013; 62(25):521-524
Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine for Adults with Immunocompromising Conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP)
MMWR, October 12, 2012; 61(40):816-9
Prevention of Pneumococcal Disease Among Infants and Children–Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine
MMWR, December 10, 2010; 59(RR11):1-18
Updated ACIP Recommendations: Prevention of Invasive Pneumococcal Disease Among Adults Using the 23-Valent Pneumococcal Polysaccharide Vaccine (PPSV23)
MMWR, September 3, 2010; 59(34):1102-6
ACIP Updates: Recommendations for Use of 20-Valent Pneumococcal Conjugate Vaccine in Children ― United States, 2023
MMWR. 2023 / 72(39);1072
Pneumococcal Vaccine for Adults Aged ≥19 Years: Recommendations of the Advisory Committee on Immunization Practices, United States, 2023.
MMWR. 2023 / 72(RR-3);1–39
Use of 21-Valent Pneumococcal Conjugate Vaccine Among U.S. Adults: Recommendations of the Advisory Committee on Immunization Practices — United States, 2024
MMWR September 12, 2024 / 73(36);793–798
Expanded Recommendations for Use of Pneumococcal Conjugate Vaccines among Adults aged ≥50 Years: Recommendations of the Advisory Committee on Immunization Practices — United States, 2024
MMWR 2025;74:1–8
Additional Federal Resources
- All current and archived ACIP Pneumococcal recommendations
- General Best Practice Guidelines for Immunization
- ACIP Pneumococcal recommendations at CDC
- CDC Pneumococcal Information for Healthcare Professionals
- Summary of Who and When to Vaccinate
- PneumoRecs VaxAdvisor Mobile App for Vaccine Providers
- Pneumococcal Vaccine Timing for Adults
- Pneumococcal Vaccine Recommendations
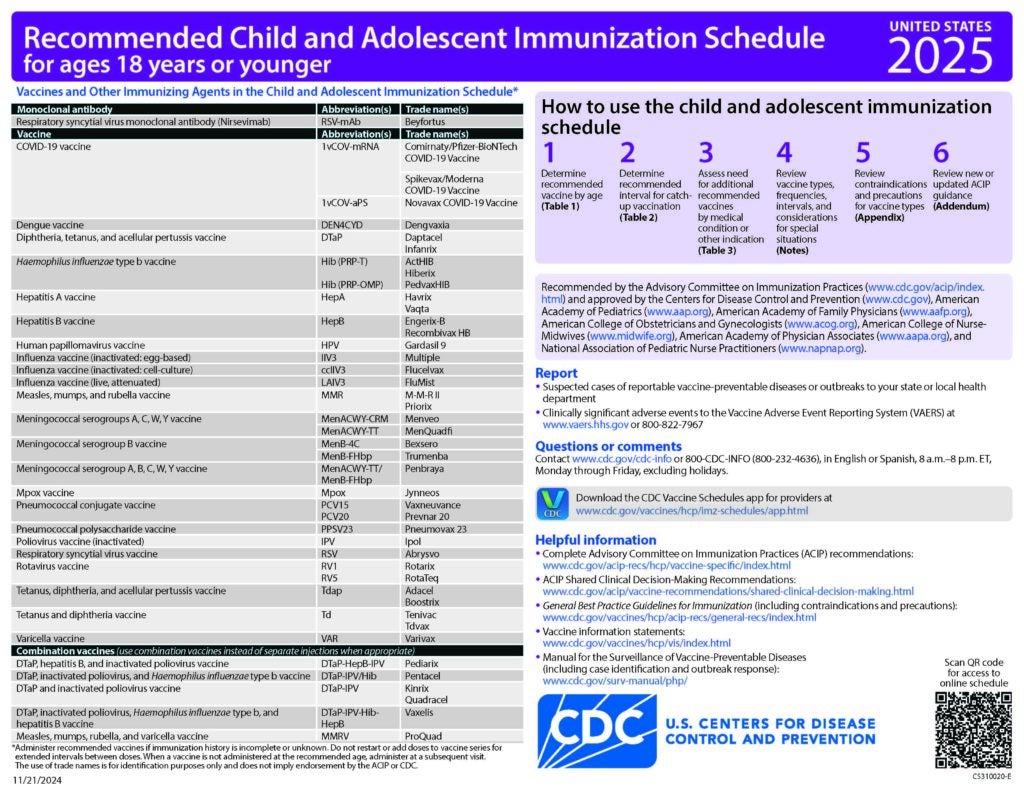
CDC Recommended Schedules
FDA Package Inserts & EUAs
Pneumococcal (PCV): Capvaxive Package Insert
Merck & Co., Inc.
Pneumococcal (PCV): Prevnar 20 Package Insert
Pfizer
Pneumococcal (PCV): Vaxneuvance (PCV15) Package Insert
Merck & Co, Inc.
Pneumococcal (PCV): Prevnar 13 Package Insert
Pfizer
Pneumococcal (PPSV): Pneumovax 23 Package Insert
Merck & Co., Inc.
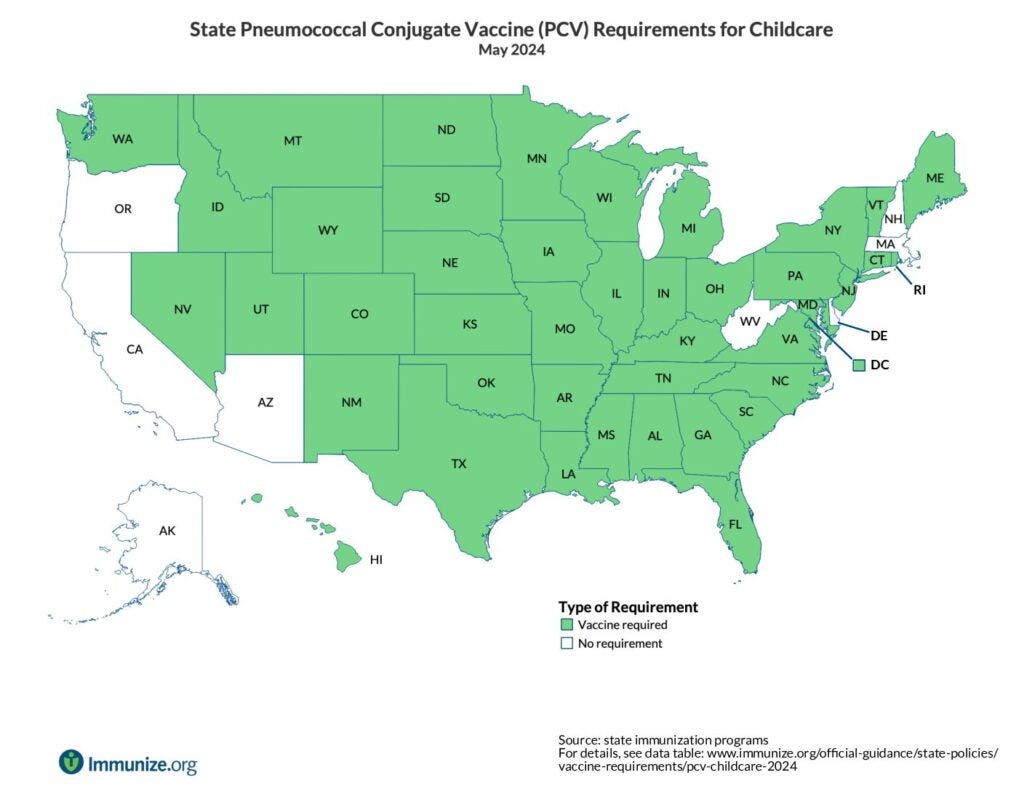
State Policies
Travel
All travelers should be up to date on routine vaccines. Depending on the destination, itinerary, and duration of travel, additional vaccines may be recommended.
CDC Resources
Travelers’ health information for healthcare providers