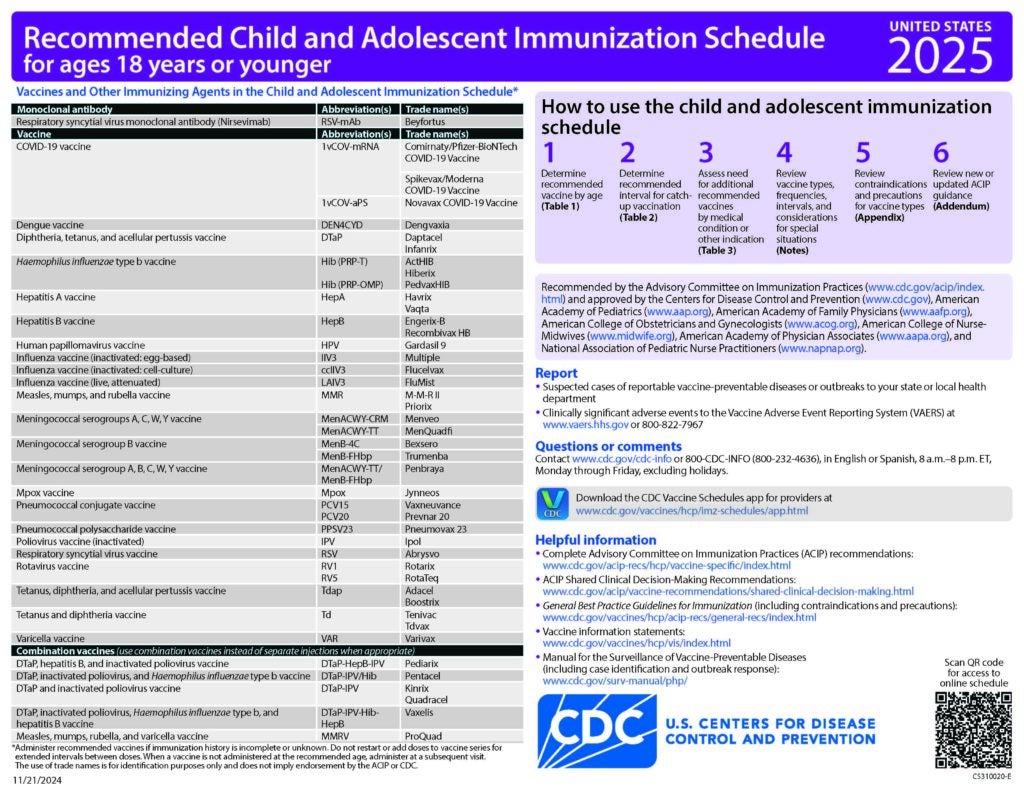
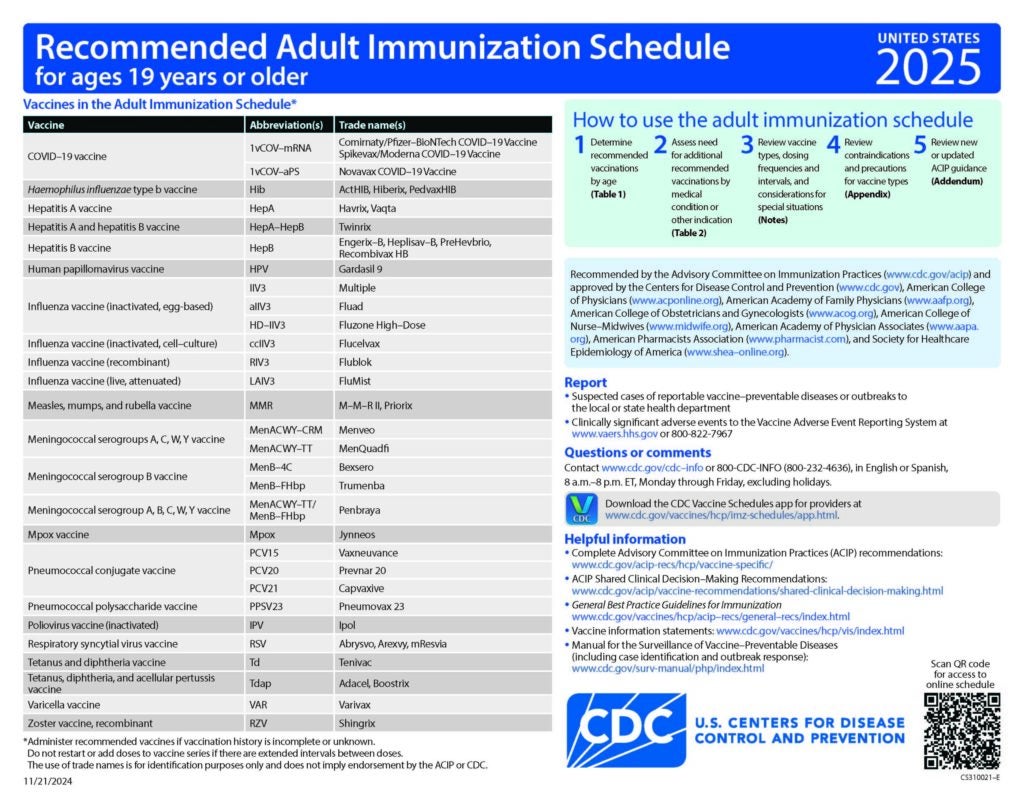
Clinical Resources
Materials for Providers
Alphabetical by Title
Standing orders for Administering Measles, Mumps, and Rubella Vaccine to Adults
Eligible healthcare professionals may vaccinate adults who meet any of the criteria on this form
CDC · FDA · State
ACIP Recommendations
Current Recommendations
Measles, Mumps, Rubella Vaccine (PRIORIX): Recommendations of the Advisory Committee on Immunization Practices—United States, 2022
MMWR, November 18, 2022, 71 (46); 1465-1470
Prevention of Measles, Rubella, Congenital Rubella Syndrome, and Mumps, 2013 — Summary Recommendations of the Advisory Committee on Immunization Practices (ACIP)
MMWR, June 14, 2013; 62(RR04):1-34
Use of Combination Measles, Mumps, Rubella, and Varicella Vaccine–Recommendations of the Advisory Committee on Immunization Practices
MMWR, May 7, 2010; 59(RR–3):1 – 16
Recommendations of the Advisory Committee on Immunization Practices for Use of a Third Dose of Mumps Virus-Containing Vaccine in Persons at Increased Risk for Mumps During an Outbreak
MMWR, January 12, 2018; 67(1); 33–37
Vaccine Information Statements
CDC Recommended Schedules
FDA Package Inserts & EUAs
Measles, Mumps, Rubella: MMR II Package Insert
Merck & Co., Inc.
Measles, Mumps, Rubella: Priorix Package Insert
GSK
Combination Vaccine: ProQuad Package Insert
Merck & Co.
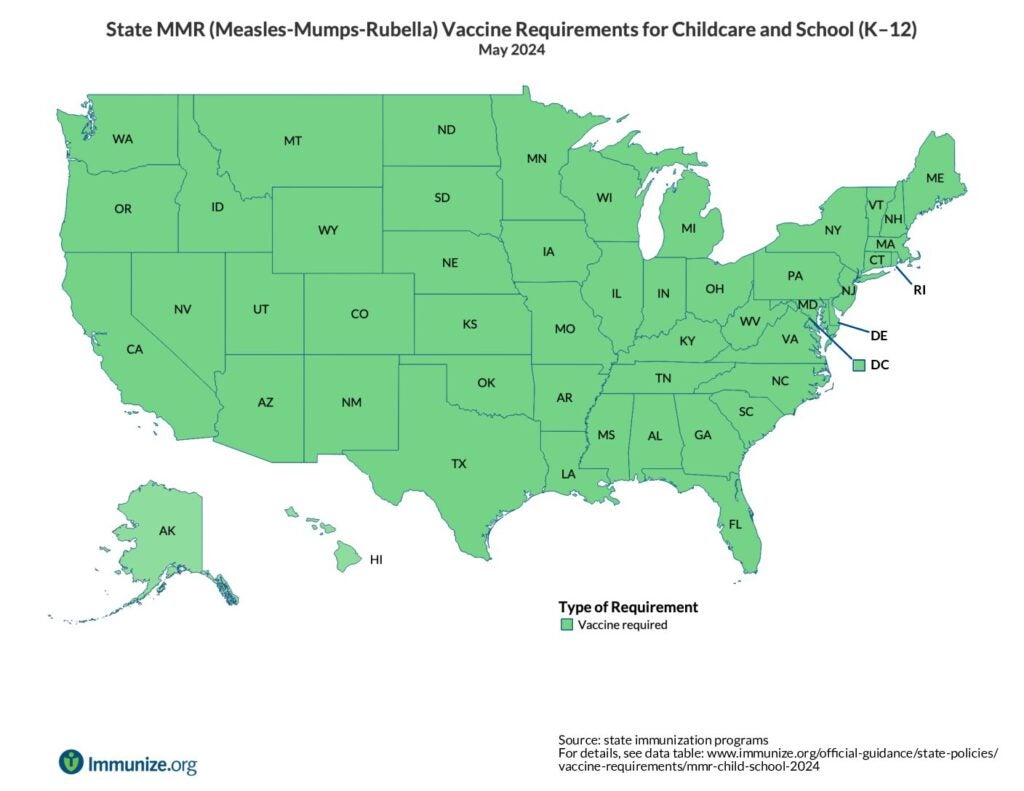
State Policies
U.S. map of MMR requirements
Travel
All travelers should be up to date on routine vaccines. Depending on the destination, itinerary, and duration of travel, additional vaccines may be recommended.
CDC Resources
Travelers’ health information for healthcare providers