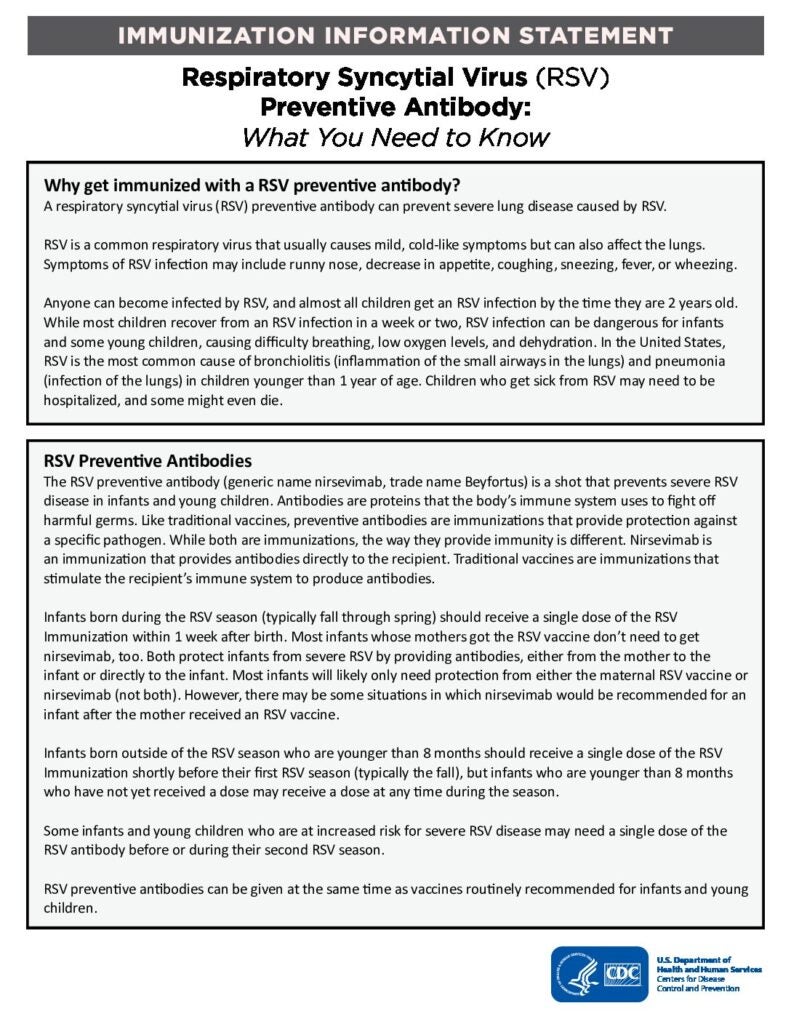
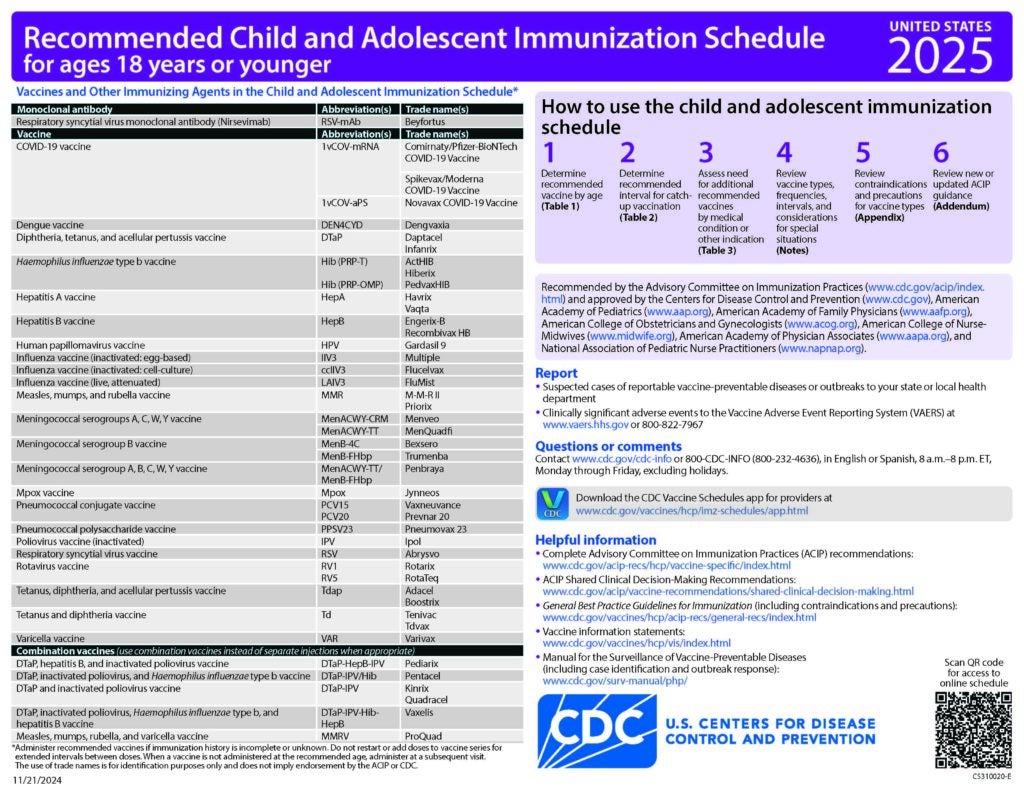
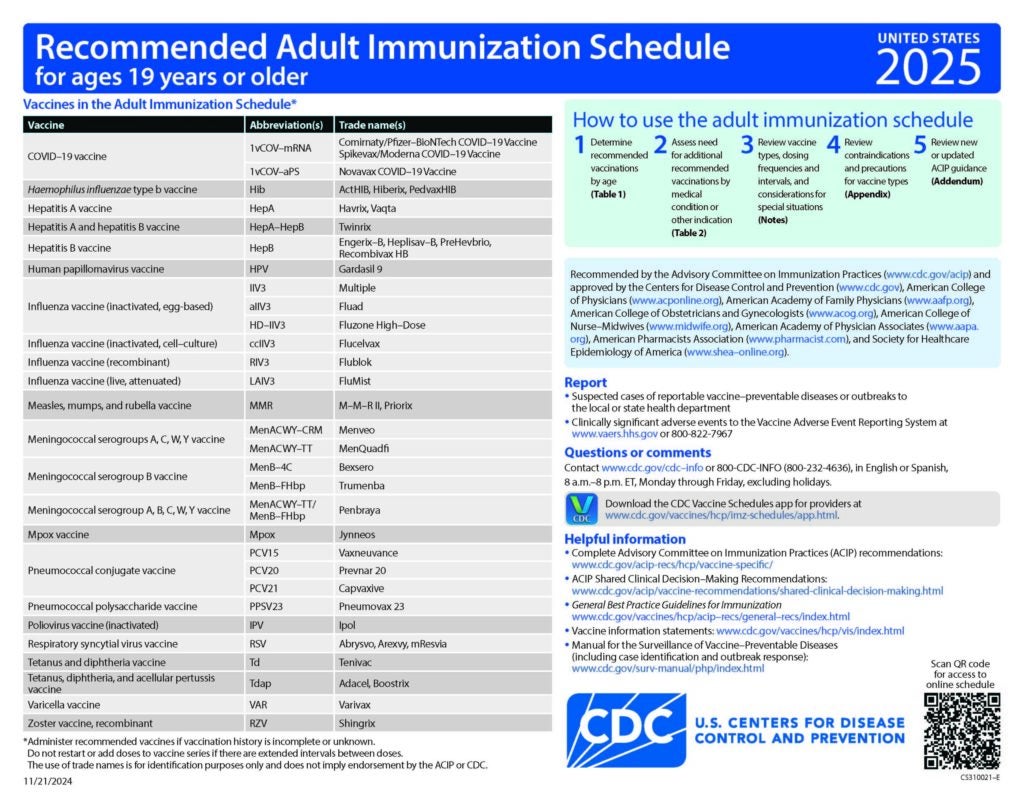
Clinical Resources
Materials for Providers
Standing Orders for Administering Nirsevimab RSV Preventive Antibody (Beyfortus, by Sanofi) to Infants
Eligible healthcare professionals may vaccinate infants who meet any of the criteria on this form
Standing Orders for Administering Pfizer Respiratory Syncytial Virus (RSV) Vaccine (Abrysvo) During Pregnancy
Eligible healthcare professionals may vaccinate pregnant persons who meet any of the criteria on this form
CDC · FDA · State
ACIP Recommendations
Current Recommendations
Use of Nirsevimab for the Prevention of Respiratory Syncytial Virus Disease Among Infants and Young Children: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023
MMWR, August 25, 2023, 72(34); 920–925
Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices
MMWR, July 21, 2023, 72 (29); 793-801
Use of the Pfizer Respiratory Syncytial Virus Vaccine During Pregnancy for the Prevention of Respiratory Syncytial Virus–Associated Lower Respiratory Tract Disease in Infants: Recommendations of the Advisory Committee on Immunization Practices — United States, 2023
MMWR, October 13, 2023, 72(41);1115-1122
Use of Respiratory Syncytial Virus Vaccines in Adults Aged ≥60 Years: Updated Recommendations of the Advisory Committee on Immunization Practices— United States, 2024
MMWR August 15, 2024 / 73(32);696-702
Additional Federal Resources
- All current and archived ACIP RSV (Respiratory Syncytial Virus) recommendations
- General Best Practice Guidelines for Immunization
- ACIP RSV (Respiratory Syncytial Virus) recommendations at CDC
- CDC RSV (Respiratory Syncytial Virus) Information for Healthcare Professionals
- RSV Vaccination for Adults 60 Years and Older
CDC Recommended Schedules
FDA Package Inserts & EUAs
RSV: Mresvia Package Insert
Moderna
RSV: Beyfortus Package Insert
Sanofi
RSV: Arexvy Package Insert
GSK
RSV: Abrysvo Package Insert
Pfizer