Clinical Resources
Materials for Providers
Alphabetical by Title
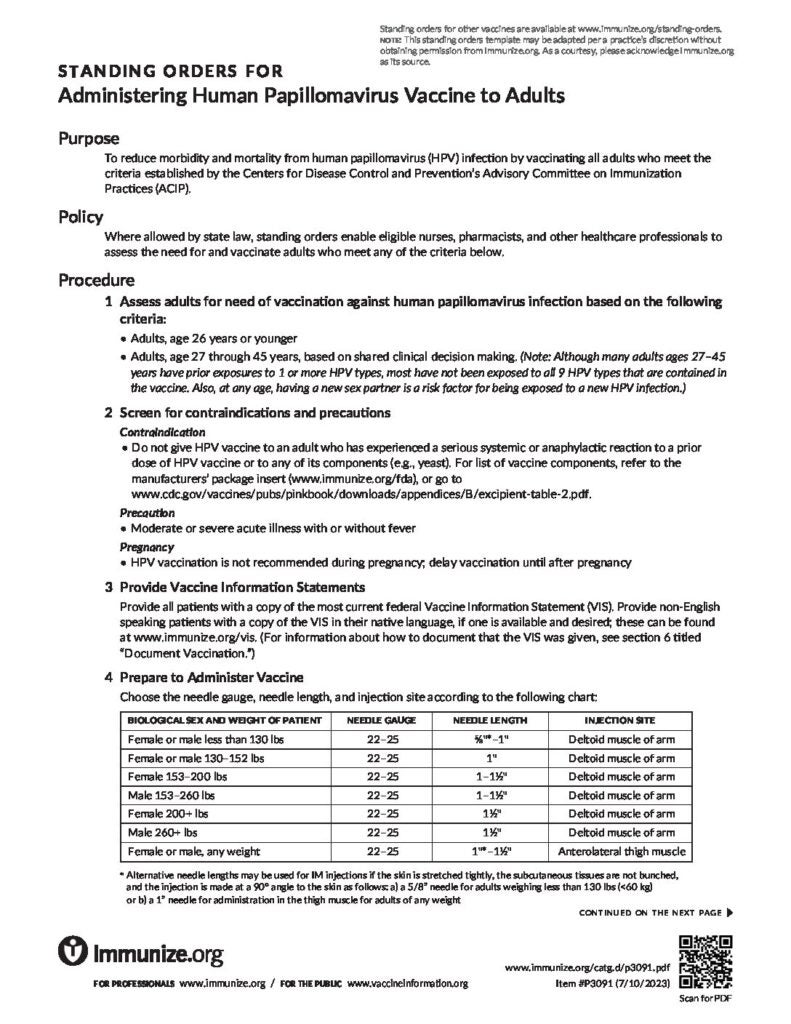
Standing Orders for Administering Human Papillomavirus Vaccine to Adults
Eligible healthcare professionals may vaccinate adults who meet any of the criteria on this form
Materials for Vaccine Recipients
Alphabetical by Title
HPV Vaccine: A Guide for Adults Ages 18-26 Years
One-page handout provides answers to common questions about HPV and HPV vaccination
- Also available in:
- Spanish
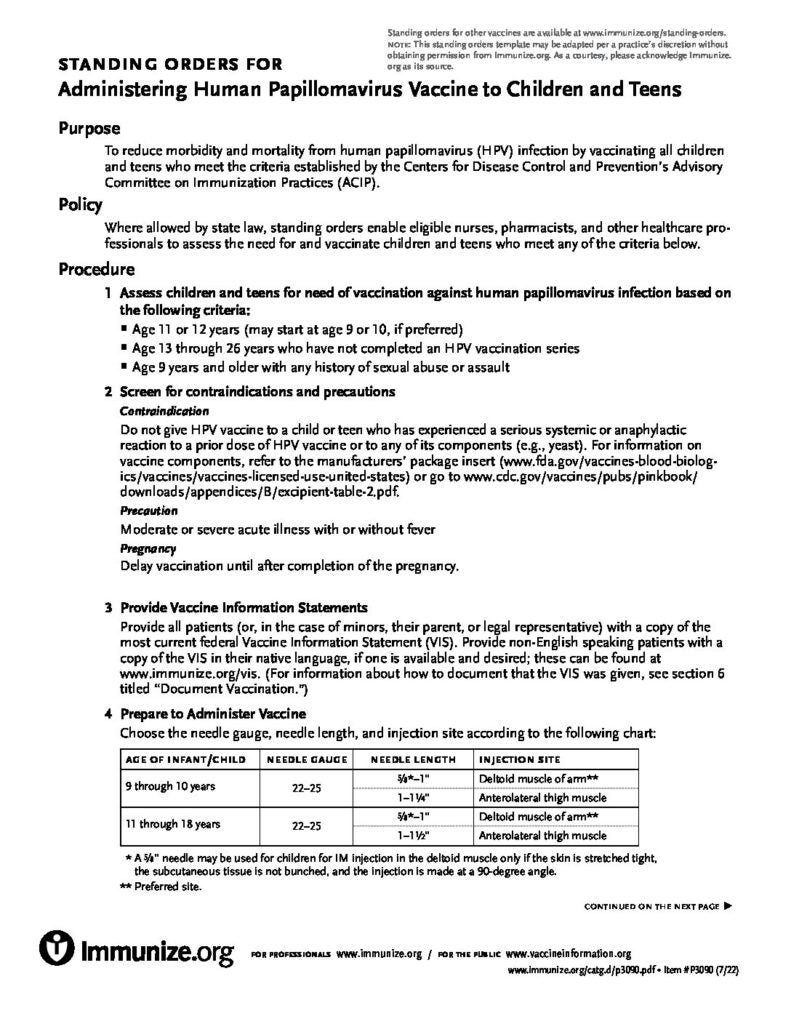
Human Papillomavirus (HPV): A Parent’s Guide to Preteen and Teen HPV Vaccination
Two-page handout provides answers to common questions about HPV and HPV vaccination
- Also available in:
- Spanish
CDC · FDA · State
ACIP Recommendations
Current Recommendations
Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices
MMWR August 16, 2019; 68(32): 698–702
Use of a 2-Dose Schedule for Human Papillomavirus Vaccination: Updated Recommendations of the Advisory Committee on Immunization Practices
MMWR, December 16, 2016; 65(49);1405-8
Use of 9-Valent Human Papillomavirus (HPV) Vaccine: Updated HPV Vaccination Recommendations of the Advisory Committee on Immunization Practices
MMWR, March 27, 2015; 64(11):300-304
Human Papillomavirus Vaccination: Recommendations of the Advisory Committee on Immunization Practices (ACIP)
MMWR, August 29, 2014; 63(RR-5):1-30
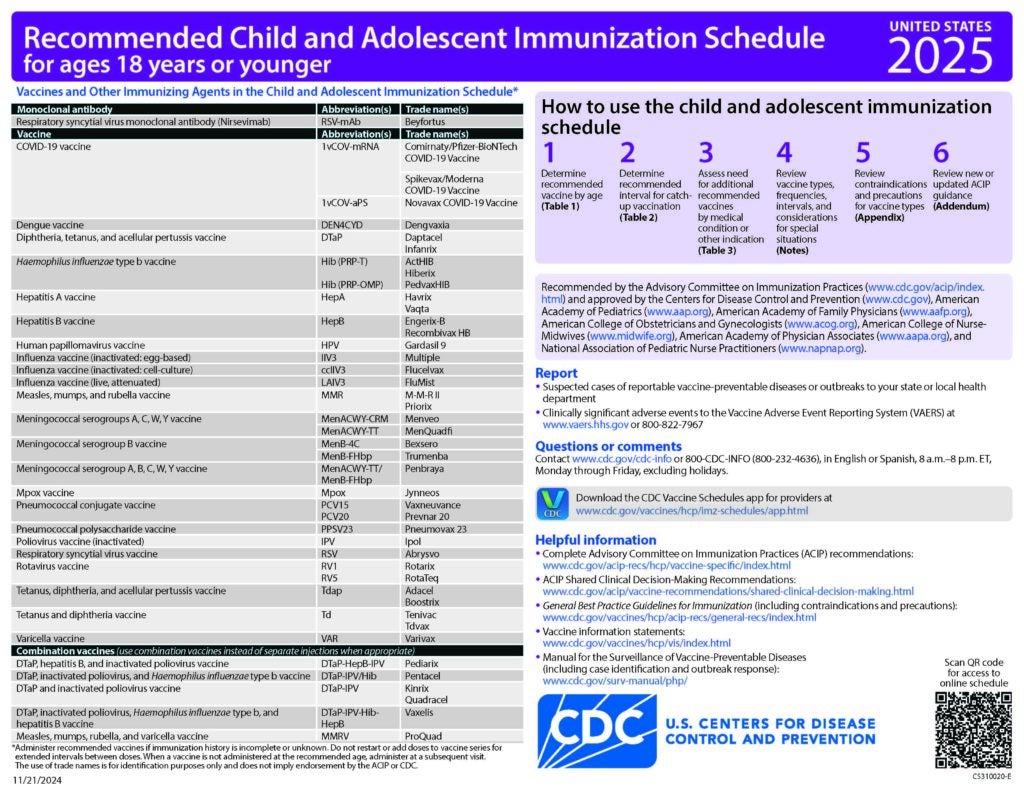
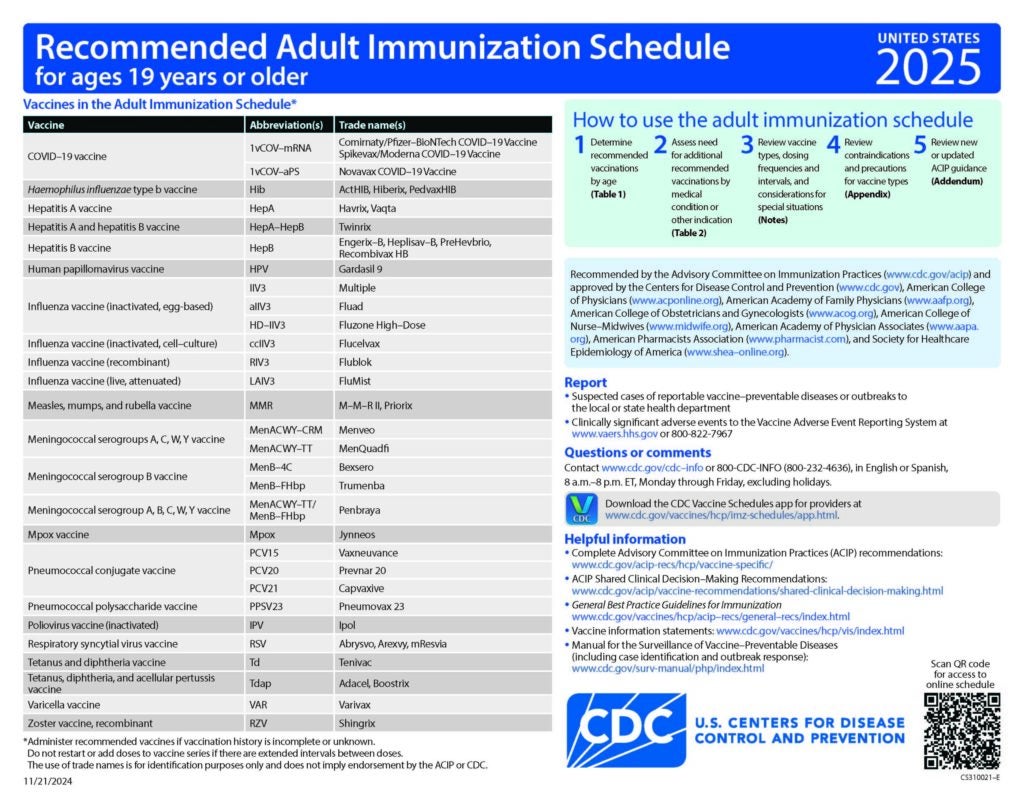
CDC Recommended Schedules
FDA Package Inserts & EUAs
Human papillomavirus (HPV): Gardasil 9 Package Insert
Merck & Co., Inc.
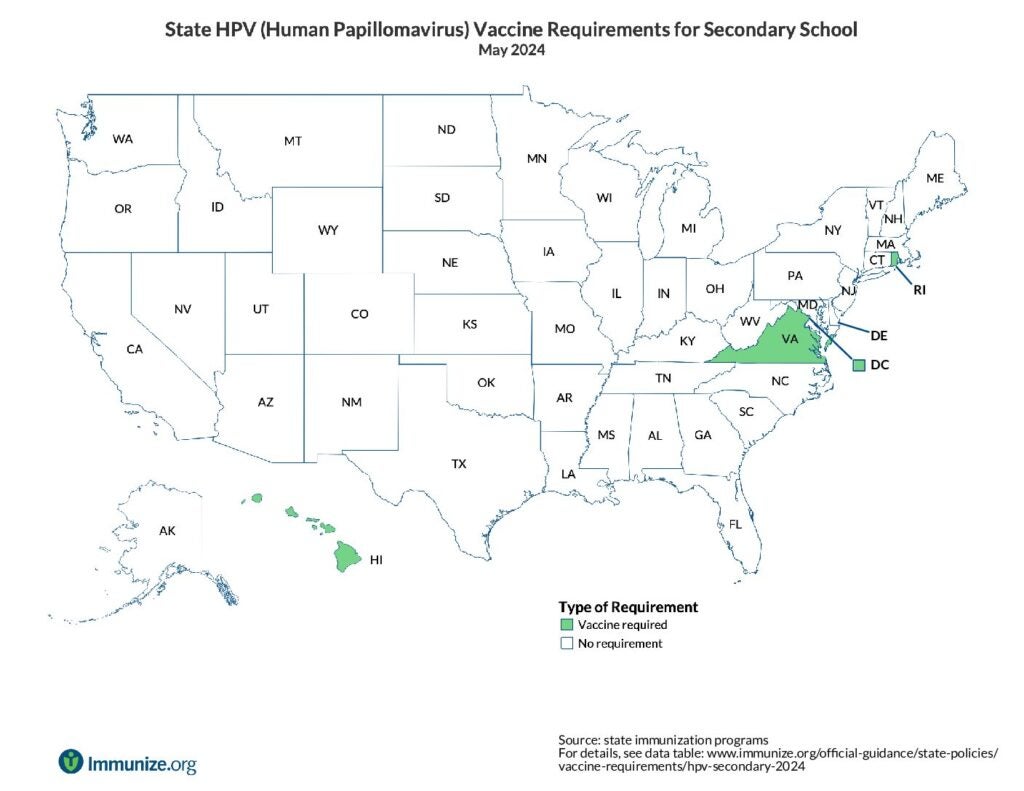
State Policies
U.S. map of HPV requirements