Clinical Resources
Materials for Providers
Alphabetical by Title
Standing Orders for Administering Varicella Vaccine to Adults
Eligible healthcare professionals may vaccinate adults who meet any of the criteria on this form
Ask the Experts
CDC · FDA · State
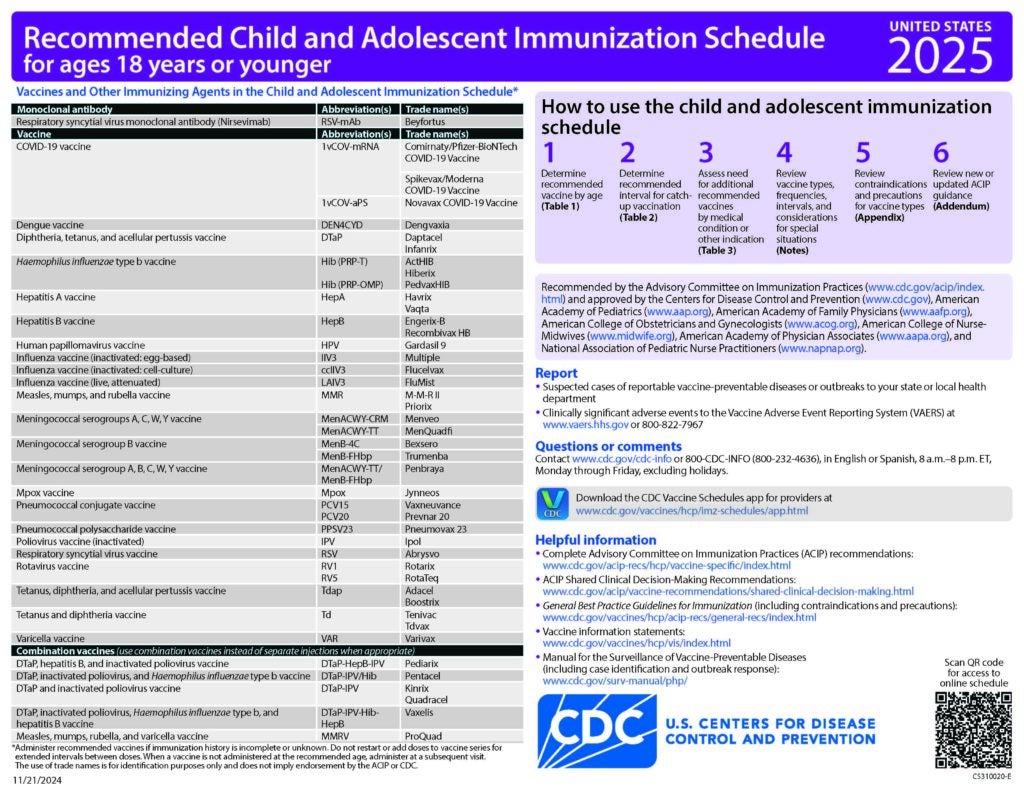
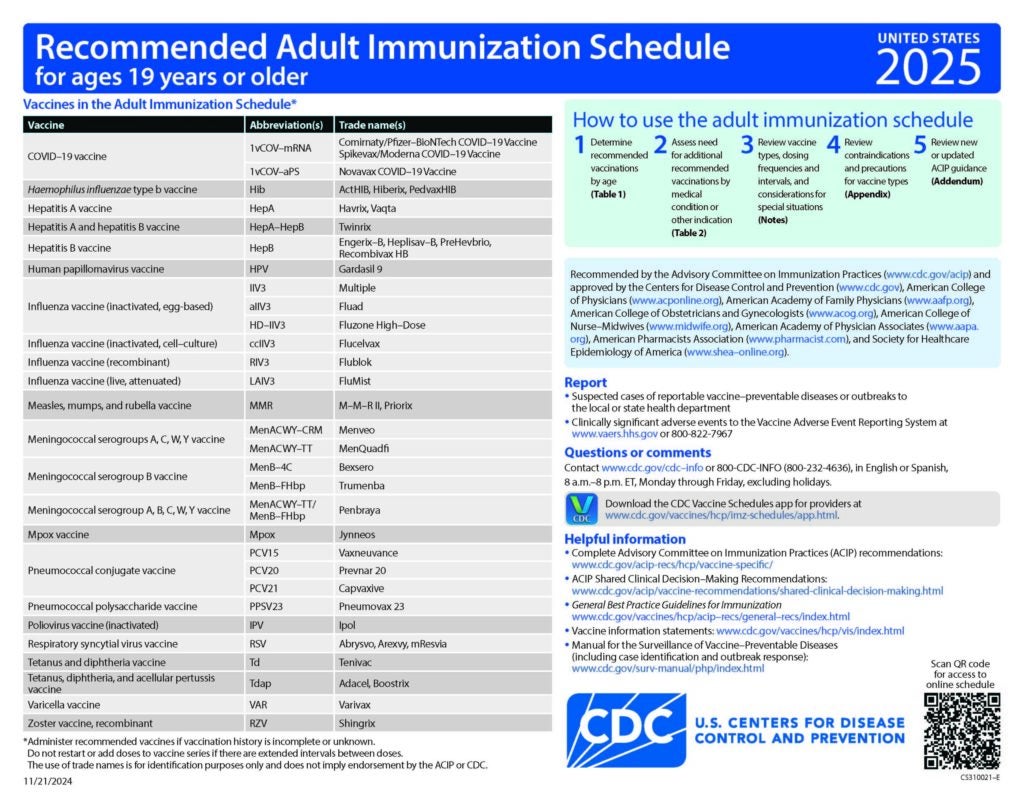
ACIP Recommendations
Current Recommendations
Use of Combination Measles, Mumps, Rubella, and Varicella Vaccine–Recommendations of the Advisory Committee on Immunization Practices
MMWR, May 7, 2010; 59(RR–3):1 – 16
Updated Recommendations for Use of VariZIG — United States, 2013
MMWR, July 19, 2013; 62(28):574-6
Prevention of Varicella Recommendations of the Advisory Committee on Immunization Practices (ACIP)
MMWR, June 22, 2007; 56(RR-4):1-40
CDC Recommended Schedules
FDA Package Inserts & EUAs
Varicella (chickenpox): Varivax Package Insert
Merck & Co., Inc.
Combination Vaccine: ProQuad Package Insert
Merck & Co.
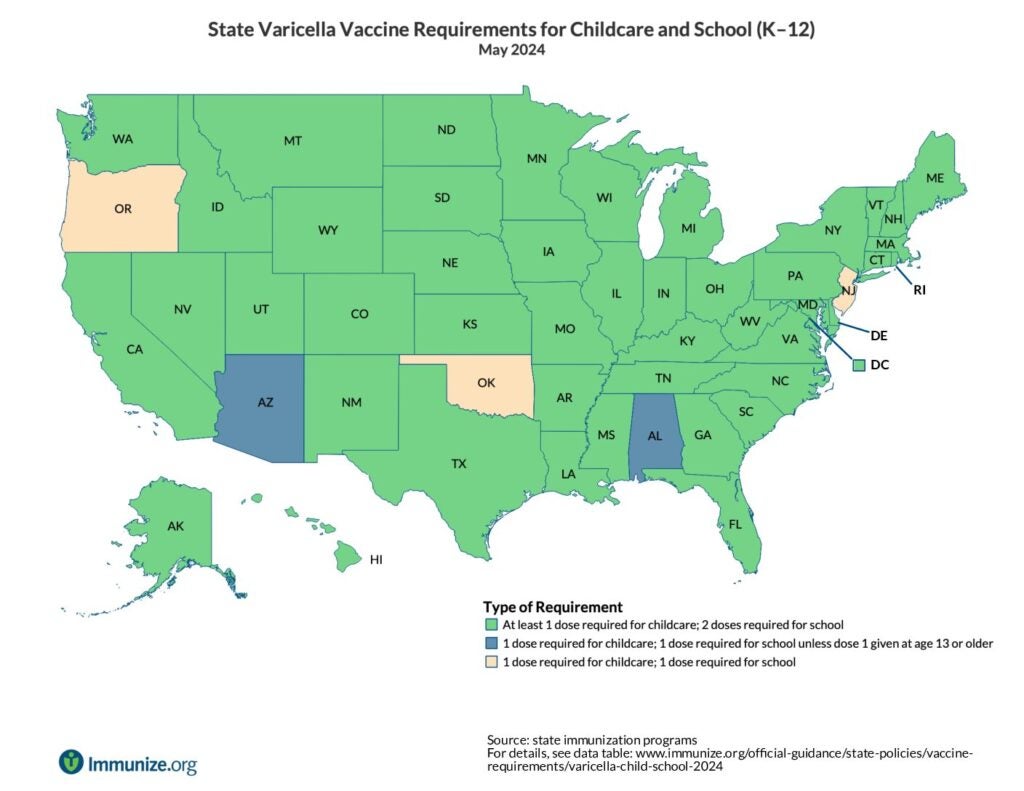
State Policies
U.S. map of varicella childcare and school requirements
Partner Resources
Governmental
CDC infographic showing the impact and success fo the varicella (chickenpox) vaccination program in the United States